Conductivity
What is conductivity?
Conductivity is how we measure the ionic content (such as chloride, nitrate, sulfate, sodium, magnesium, calcium, or iron) in a body of water by measuring the water’s ability to conduct electricity.

Why is conductivity important?
Every body of water has a unique conductivity level based on its bedrock. Establishing a baseline is important, as some bodies of water have naturally high levels due to their geology and geography.
What does a conductivity measurement mean?
Conductivity can be used as an early warning system for potential problems that warrant further testing. If we see a variance (either lower or higher) from our baseline readings, we know something may have gone wrong. While lab testing is usually the only way to determine what is causing the reading, higher readings could indicate a pollution event has taken place.
What about ocean water? Conductivity for ocean water is about 55,000 µS. While some people test conductivity in seawater, many meters won’t read that high. Many protocols choose to measure salinity instead. Salinity is measured in parts per thousand, with the average ocean reading measuring 35 ppt. Like all tests, we can convert readings, although conductivity measures everything that conducts electricity, while salinity only measures salt.
Some common values
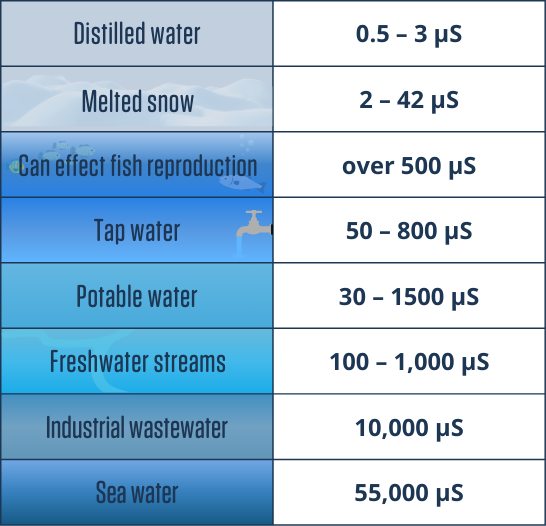
For example, most readings for the Ottawa River are below 100 µS, but near a storm sewer, conductivity values could be over 2,000 µS (or 2.0 mS).
Ask an expert
Conrad Gregoire, Chemist, introduces Conductivity
Water Rangers testing protocol
Conductivity is measured by placing a conductivity probe in the water and measuring the flow of electricity between the electrodes.

Note: old models have a cap you need to remove. If you purchased your meter after 2021, you will not have a cap.
Research
- Long-term reproducibility: How well does this probe stay accurate? (Dr. Conrad Gregoire)
- Comparison to professional probes: How well does our testkit compare to professional equipment? (Carleton University, Conservation Authorities)
- Turn on the meter by pressing the top button.
- Dip the meter into the water collected in your sample cup. Do not dunk the whole device in, as the battery is near the top. Hold in the water for 2 minutes, swishing it around lightly. Continue until both values remain steady for 30 seconds.
- Tip: If you get a reading of 10 or less, you have not removed the cap or you have taken the sensor out of the water before reading) Read the measurements. We record in µS/cm (microsiemens per centimetre), so check the units. If you get a reading like 1.3, it converts the measure to mS (millisiemens), and you must multiply 1.3 by 1000 and record 1,300 in your form.
- Don’t forget to turn off the device after use to preserve battery life (top button).
How to test conductivity
Taking care of your conductivity meter
Since the probe is conducting an electric current, it is so important to keep the electrode clean. Rinse electrodes in distilled water often, especially if you are taking samples where readings are high or if the area is polluted.
Calibration
You should calibrate your device before every season or if you suspect damage. Learn how to calibrate your meter here. One pouch of calibration solution is provided to those who have purchased a testkit. For additional pouches, visit our store.
Ask an expert
Conrad Gregoire, Chemist, how to test and use a reference to make sure you’re accurate.
Get a conductivity meter here or as part of a testkit:
-
 Compact Freshwater TestkitCAD$159.00
Compact Freshwater TestkitCAD$159.00 -
 Conductivity meterCAD$77.00
Conductivity meterCAD$77.00 -
 Freshwater conductivity calibration solutionCAD$5.50
Freshwater conductivity calibration solutionCAD$5.50
Contributing to the community!
Water Rangers is citizen-scientist led. So, if you have any questions, ideas, or notice any errors, please tell us!
