Total dissolved solids (TDS)
What are total dissolved solids?
Total dissolved solids (TDS) are made up of inorganic salts (magnesium, potassium, calcium, bicarbonates, sodium, chlorides, and sulfates) and some small amounts of organic matter that are dissolved in water.
Why are total dissolved solids important?
A high concentration of dissolved solids on its own is usually not a health hazard. In fact, the mineral water you buy in stores have naturally elevated levels of TDS. However, a high concentration of TDS can be an indicator that harmful contaminants, such as iron, manganese, sulfate, bromide and arsenic, may be present in the water due to human pollution. It also affects the hardness of the water.
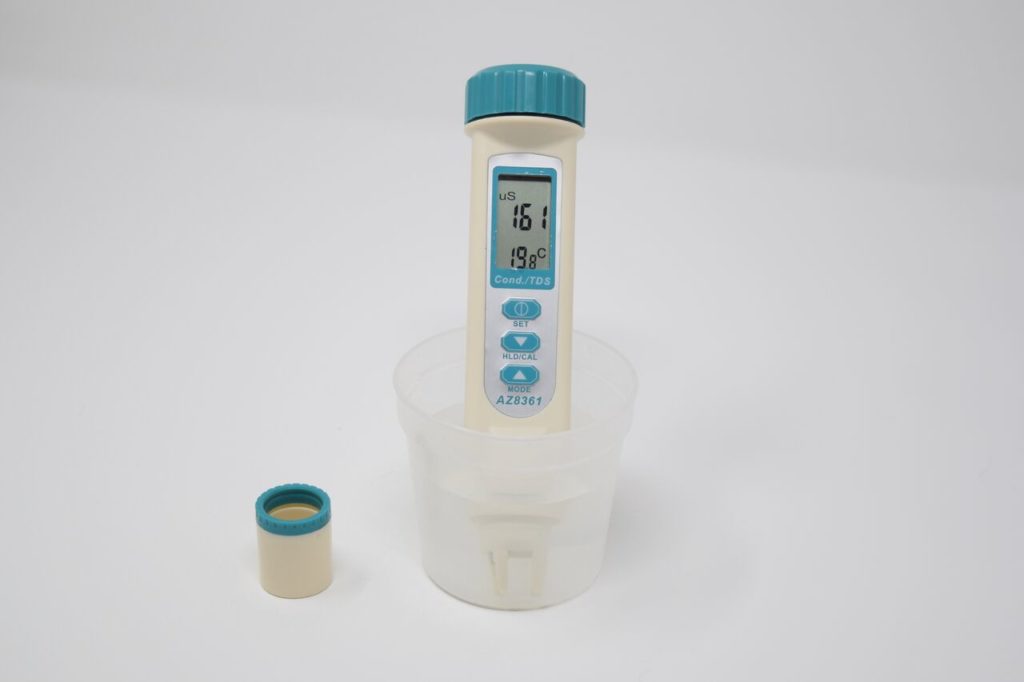
What does a measurement mean?
These are normally only measured in freshwater systems. Salinity measures some of the ions that are included in the definition of TDS. According to Health Canada: “An aesthetic objective of ≤500 mg/L has been established for TDS in drinking water. At higher levels, excessive hardness, unpalatability, mineral deposition and corrosion may occur. At low levels, however, TDS contributes to the palatability of water.”
Water Rangers Protocol
The Water Rangers conductivity meter can also give you readings for TDS, however, since it’s a straight conversion, we do not record it. Other protocols may use TDS instead of conductivity. The other main test used to measure TDS is gravimetric analysis.
Further reading
Contributing to the community!
Water Rangers is citizen-scientist led. So, if you have any questions, ideas, or notice any errors related to conductivity, please tell us!
