Water dynamics: temperature, salinity and density
Did you know that water temperature plays a vital role in how all of our water systems work? In this mini lesson, students will explore the relationship between water temperature, salinity, and density, and their impact on water systems. Through engaging activities, they will investigate the formation of layers in water bodies, the effects of temperature on nutrient mixing, and the connection to stratification and ocean currents.
Recommended grades
This activity is recommended for grades 7 to 9. We’ve included tips and tricks to adapt the activity for a wide range of students throughout the lesson.
Learning outcomes
- Use scientific tools to accurately measure temperature.
- Apply knowledge of density, salinity, and temperature to predict and interpret changes in water systems.
- Recognize and appreciate the significance of temperature in maintaining the health and functioning of lakes and oceans.
Lesson
Background
Water temperature and why is it important?
Water temperature tells us how hot or cold water is. It is a crucial factor in understanding aquatic environments and has a significant impact on other measured parameters like pH, dissolved oxygen, and conductivity. It plays a vital role in determining water’s ability to support life, absorb gases, and take in nutrients by affecting water density. Different aquatic species also have their preferred temperature zones. Some plants thrive in warmer waters, while certain fish, like trout or salmon, prefer cooler streams.
What is salinity?
Salinity refers to the measurement of salts dissolved in water. It is important to note that this salt is not the same as the salt we use for seasoning food. Salinity in water comes from sources like rocks and soil and becomes less concentrated when ice melts or rivers flow.
What is density?
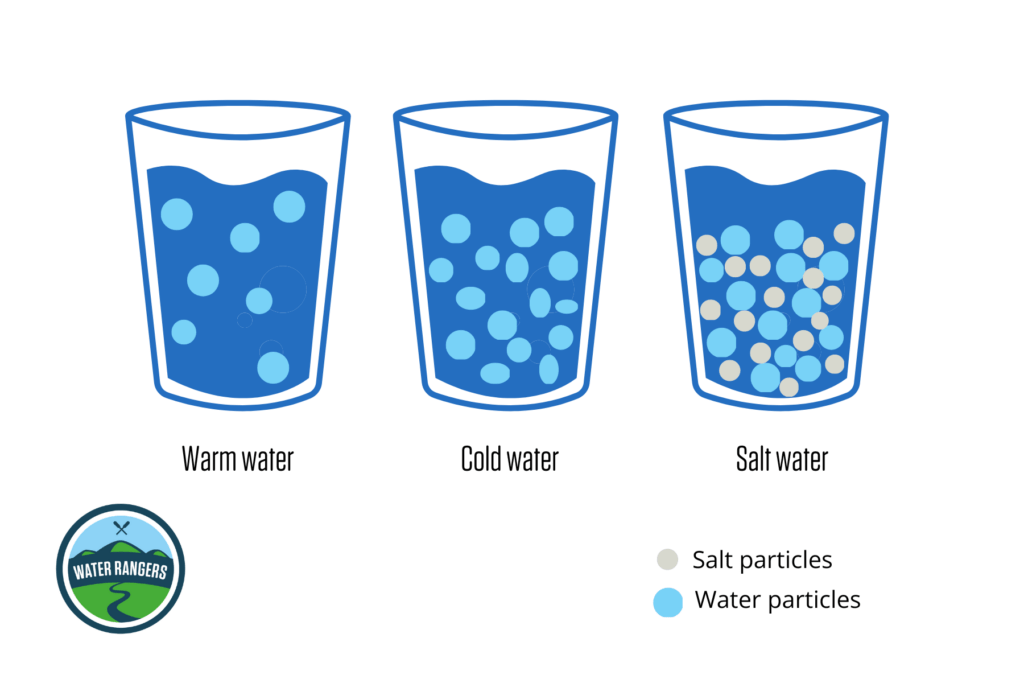
Density is a measure of how tightly or loosely particles are packed together in a substance. In water, warmer temperatures cause the particles to spread out, making the water less dense. Conversely, colder water has tightly packed particles, resulting in higher density. When it comes to saltwater, the presence of salt particles increases its density. Saltwater contains both salt and water particles, making it denser than freshwater.
When water is denser, it contains more particles and is heavier, causing it to tend to sink. On the other hand, lighter and less dense water rises up. Temperature and salinity are the two factors that influence water density in the ocean, affecting water movement. Understanding density allows us to interpret the intricate dance of water molecules that shape patterns in aquatic ecosystems!
In the classroom
Duration: 45 minutes
Objective: Students will explore salinity and density by conducting hands-on activities to observe the effects of saltwater and temperature on water density.
Groups: We recommend groups of 3-5 for this activity.
Materials needed
- Table salt
- Dark food coloring (blue or black)
- Ice tray (2 ice cubes per group)
- Tablespoons
Per group:
- 3 glasses of room temperature (warm) tap water
- 100 mL of room temperature tap water with dark food coloring
Steps
Pre-class preparation
- Add several drops of food coloring to water and freeze overnight to create dark-colored ice cubes.
- Freeze at least 2 ice cubes per group.
- Prepare room temperature water for the students by either leaving a pitcher of water out for a few hours or setting up their stations with one half-full glass (glass A) and 2 full glasses (glass B and C) of room temperature water before starting.
Exploring salinity and density
Teacher tip!
This part can be tricky! Watch this video for more details and demonstrate it to your class before performing.
Ocean science in your kitchen- Instruct each group to label the glasses A, B, and C for control.
- In glass A, mix half a glass (150 ml) of room temperature water with one heaping tablespoon of salt. Mix the water well until the salt is dissolved.
- Ask students to predict what will happen when they mix the colored tap water with the saltwater.
- By tilting the glass with saltwater, gently pour the colored tap water into the glass, letting it run on the side to avoid splashes.
- Ask students to observe and note any changes in color and movement when the colored tap water meets the saltwater.
- What is happening? Saltwater has a higher density than tap water because salt particles are packed with the water particles. When the blue tap water is added, it floats on top of the saltwater. The denser saltwater sinks to the bottom of the glass, while the less dense tap water floats at the top. Some of the colored tap water may mix with the saltwater in the middle.
Exploring salinity, temperature, and density
- Have the groups fill glass B and C with room temperature water.
- In glass B, add a heaping tablespoon of salt into the water and mix until it is dissolved. This makes glass B more dense than glass C.
- Pass two ice cubes to each group. Instruct students to place one ice cube in glass C as a control. Ask them to write their predictions before dropping the ice cube and then note their observations of how the water moves and changes color.
- What is happening? Cold water is more dense than warm water, so it sinks. However, the cold water mixes with the warm water in the glass and begins to warm up, becoming less dense and starting to rise again.
- Repeat the experiment with glass B, writing predictions and observations
Suggestions for enrichment
Check out the ‘Ocean connections’ section to explore the concept of ocean currents and ocean stratification. You can discuss convection currents, how rising temperatures are affecting ocean currents, and how melting ice changes temperature and salinity. You can also add red food coloring and hot water to enhance the experiment and create more distinct layers – just like the ocean!
Example video using red and blue food coloringWhat is happening?
The glass is filled with salty water, which is more dense than tap water. The cold colored water does not sink as much. Even though cold water is more dense than warm water, it can sit on top of the warm water because it has a high salinity
Discussion and reflection
- What did you observe when the colored tap water was poured into the saltwater? How did the colors and movement change?
- Why did the colored tap water float on top of the saltwater? What does this tell us about the density of saltwater compared to tap water?
- What role does salinity play in the density of water? How does the addition of salt affect the density of water?
Outdoors: Exploring temperature with different samples
Duration: 30 minutes
Objective: Students will collect water samples from various locations around a local water body and use a thermometer and conductivity meter to measure the temperature. They will analyze the variations in temperature and discuss the factors that contribute to these variations.
Groups: We recommend groups of 4-5 for this activity.
Materials needed

- Thermometer
- Conductivity meter
- Sample cups
- Reacher sticks
- Notebooks
Do you have your Education testkit?
All materials for outdoor activities can be found in the Water Rangers education testkit.
Education testkitThis activity involves looking out for different spots around your local water body to take various samples from. Please refer to the safety tips in your educator’s guide, as well as our testing location guide and general tips to choosing a sample location. Always make sure to:
- Fully extend the arm of the reacher stick so students can easily access the water easily.
- Choose a site away from fast moving water where youth can easily reach the water.
- If the water is deeper than 0.5m, students must wear a lifejacket.
- Be extra careful around cold water.
Steps
Physical observations
- Ask students to record in their notebooks the weather from the previous day and the current weather.
- Instruct students to observe the testing site and identify different water sources that could have different temperatures. These could include puddles, shallow and deeper locations, areas in the sunshine, and areas with or without vegetation.
- Use your discretion to determine if samples from students’ suggested locations can be collected safely.
Recording air temperature
- Based on their physical observations, ask students to guess the air temperature.
- Provide each group with a thermometer.
- Instruct students to hang the thermometer under a tree or another shady spot where the testing is being conducted.
- Ensure the thermometers are at least 1.5 meters off the ground.
- Leave the thermometers there for 5 minutes before reading the values. You can choose to record the values after completing the water sampling.
Preparing to collect a sample
- Give each group a reacher stick, sample cup, and conductivity meter.
- Hook the reacher stick onto the sampling cup and ensure it holds tightly just below the lip of the sample cup.
- If you have a clamp reacher stick, extend the clamp and insert the top end of the cup into the clamp.
- Fully extend the reacher stick.
- Remind students to rinse the sample cup three times and ensure their hands are dry before using the strips.
- Turn on the conductivity meter by pressing the top button.
Collecting the sample(s)
- Based on the agreed sampling locations, ask students to predict whether they think the water will be warmer or cooler and to guess the units.
- For each approved location, have each group collect a sample and place the conductivity meter in the sample cup water.
- Do not dunk the whole device as the battery is near the top. Hold it in the water for 2 minutes, swishing it around lightly until both values on the meter remain steady for 30 seconds.
- The smaller lower value on the meter will show the temperature in degrees Celsius.
- Repeat this process for each location, encouraging students to try collecting samples from different depths (surface and deeper samples).
- Return to the thermometers and record air temperature.
Reflection and discussion
Teacher tip!
The discussion and reflection section can be used to evaluate students’ knowledge.
- What factors do you think contributed to the temperature variations in the samples?
- How did the air temperature affect the water temperature? How does this change with freshwater and saltwater bodies?
- How does water depth change the temperature of the water? What do you think this means for the aquatic life in the water body?
What is happening?
When you go swimming and go to the bottom, you may notice that it’s cold down there! Like the classroom experiment, warm water will rise to the surface of the water because it is less dense, while cold water sinks to the ground. In natural water bodies like lakes and oceans, this creates layers of water with different temperatures.
Air temperature also affects the temperature of water bodies. When the air is warm, it can transfer heat to the water, causing the water temperature to rise. And when the air is cold, it can cool down the water. The effect of air temperature on water temperature can be different in freshwater and saltwater bodies. Freshwater tends to change temperature more quickly in response to changes in air temperature because it has a lower density compared to saltwater. Saltwater, such as in the ocean, is denser and can hold heat more effectively, so its temperature changes more slowly.
The temperature variations caused by air temperature and water depth have important implications for aquatic life. Some organisms prefer warmer water near the surface, while others are adapted to the colder depths. The mix of different temperatures in a waterbody lets diverse species coexist.
Ocean connections
Ocean currents
The relationship between salinity, temperature, and density influences ocean currents. Differences in salinity and temperature affect how water moves vertically: denser water sinks, while less dense water rises. Horizontal movements are influenced by factors like wind and the Earth’s rotation. Wind pushes surface waters, forming surface currents, while the Earth’s rotation causes water to deflect, creating circular patterns called gyres.
Ocean stratification
Ocean stratification refers to the layering of water in the ocean based on temperature and salinity. Warmer surface waters stay on top of colder deep waters, creating different zones in the ocean. Ocean stratification affects the distribution of heat, nutrients, and oxygen in the ocean, which also impacts where marine life can be found.
Climate change is making ocean stratification stronger. As the Earth’s atmosphere gets warmer, the ocean absorbs more heat, causing the surface waters to warm up. This strengthens ocean stratification and makes it harder for water to move between different layers. This can reduce the availability of nutrients in the surface waters, which affects the growth of plants and animals in the ocean. These changes also affect the flow and strength of ocean currents, which can have effects on weather, ecosystems, and the overall climate.
Real world connections
Although most lakes’ water mix completely during a typical year, not all do! In Val-Des-Monts, Quebec, Water Rangers’ research on Lake McGlashan revealed that Lake McGlashan is a meromictic lake. This means that distinct layers of water with varying properties do not mix thoroughly. This phenomenon impacts the lake’s ecosystem, influencing factors like dissolved oxygen levels and aquatic life distribution.
Explore more about meromictic lakes and their dynamics in our blog postAdditional resources
Lesson resources
Long story shorts: what is ocean stratification?
Introduction to lake stratification
Turnover, lake mixing and stratification
Additional activities
Warm water rises and cold water sinks
Glossary
Control sample: A sample used as a baseline or reference to compare with other samples. In experiments, it helps understand the initial conditions before any changes or treatments
Currents: The steady movement of water in a specific direction.
Particles: Tiny pieces of matter that make up everything around us.
Volume: The amount of space something takes up.
